how to find oxidation number
OXIDATION NUMBERS CALCULATOR
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'Calculate' (for example: Ca2+, HF2^-, Fe4[Fe(CN)6]3, NH4NO3, so42-, ch3cooh, cuso4*5h2o).
The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. The oxidation number is synonymous with the oxidation state. Determining oxidation numbers from the Lewis structure (Figure 1a) is even easier than deducing it from the molecular formula (Figure 1b). The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Bonds between atoms of the same element (homonuclear bonds) are always divided equally.
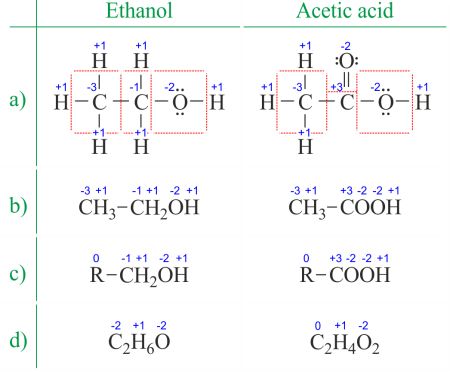
Figure 1. Different ways of displaying oxidation numbers of ethanol and acetic acid. R is an abbreviation for any group in which a carbon atom is attached to the rest of the molecule by a C-C bond. Notice that changing the CH3 group with R does not change the oxidation number of the central atom. → Download high quality image
When dealing with organic compounds and formulas with multiple atoms of the same element, it's easier to work with molecular formulas and average oxidation numbers (Figure 1d). Organic compounds can be written in such a way that anything that doesn't change before the first C-C bond is replaced with the abbreviation R (Figure 1c). Unlike radicals in organic molecules, R cannot be hydrogen. Since the electrons between two carbon atoms are evenly spread, the R group does not change the oxidation number of the carbon atom it's attached to. You can find examples of usage on the Divide the redox reaction into two half-reactions page.
Rules for assigning oxidation numbers
- The oxidation number of a free element is always 0.
- The oxidation number of a monatomic ion equals the charge of the ion.
- Fluorine in compounds is always assigned an oxidation number of -1.
- The alkali metals (group I) always have an oxidation number of +1.
- The alkaline earth metals (group II) are always assigned an oxidation number of +2.
- Oxygen almost always has an oxidation number of -2, except in peroxides (H2O2) where it is -1 and in compounds with fluorine (OF2) where it is +2.
- Hydrogen has an oxidation number of +1 when combined with non-metals, but it has an oxidation number of -1 when combined with metals.
- The algebraic sum of the oxidation numbers of elements in a compound is zero.
- The algebraic sum of the oxidation states in an ion is equal to the charge on the ion.
Assigning oxidation numbers to organic compounds
- The oxidation state of any chemically bonded carbon may be assigned by adding -1 for each bond to more electropositive atom (H, Na, Ca, B) and +1 for each bond to more electronegative atom (O, Cl, N, P), and 0 for each carbon atom bonded directly to the carbon of interest. For example:
- propene: CH3-CH=CH2
- lauric acid: CH3(CH2)10COOH
- di-tert-butyl peroxide: (CH3)3COOC(CH3)3
- diisopropyl ether: (CH3)2CH-O-CH(CH3)2
- dibenzyl sulfide: (C6H5CH2)2S
- cysteine: HO2CCH(NH2)CH2SH
Citing this page:
Generalic, Eni. "Oxidation numbers calculator." EniG. Periodic Table of the Elements. KTF-Split, 22 Jan. 2021. Web. {Date of access}. <https://www.periodni.com/oxidation_numbers_calculator.php>.
how to find oxidation number
Source: https://www.periodni.com/oxidation_numbers_calculator.php
Posted by: velozbeted1955.blogspot.com

0 Response to "how to find oxidation number"
Post a Comment